Phase rule - Soritsu (English spelling)
|
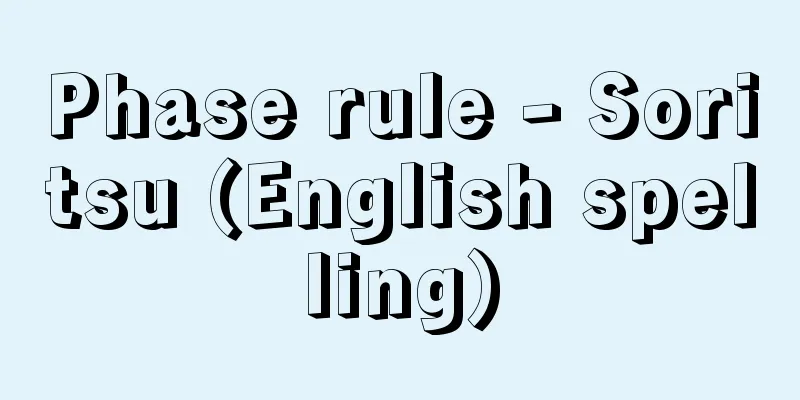
This is the law that determines the degrees of freedom when there is multiphase equilibrium, that is, equilibrium between two or more phases. It was published by American J. W. Gibbs between 1876 and 1878, and is also known as the Gibbs phase rule. The number of independent state variables (pressure, temperature, and concentrations of component substances) required to determine the state of a system in equilibrium is called the degrees of freedom. If the degrees of freedom are f , then when a system consists of n components and is in equilibrium as p phases, then: [Toda Genjiro and Nakahara Katsunori] [Reference] | | | | |Source: Shogakukan Encyclopedia Nipponica About Encyclopedia Nipponica Information | Legend |
|
多相平衡、すなわち二つ以上の相の間で平衡が成立しているとき、その自由度を決める法則をいう。1876年から1878年にかけてアメリカのJ・W・ギブスによって発表され、ギブスの相律ともいう。平衡にある系の状態を決めるのに必要な独立の状態変数(圧力、温度および成分物質の濃度)の数を自由度というが、その自由度をfとするとき、n個の成分からなり、p個の相として平衡にある場合、 [戸田源治郎・中原勝儼] [参照項目] | | | | |出典 小学館 日本大百科全書(ニッポニカ)日本大百科全書(ニッポニカ)について 情報 | 凡例 |
<<: Prime Minister's Office - Sorifu
Recommend
oogonium
…they produce eggs inside and are involved in sex...
Demiurge (English spelling)
A Greek word meaning maker or craftsman. (1) In Ho...
Felis libyca; Caffre cat; African wildcat
Carnivora, Felidae. Body length 70cm, tail length ...
Torbernite (torbernite)
Also called torbanite. A type of uranium mica, a m...
Canal
Canal is a long, narrow, straight channel that is ...
Relaxation method - relaxation method
…Special techniques are required to measure the r...
Kokonoe [town] - Kokonoe
A town in Kusu County in western Oita Prefecture. ...
New Britain [island] - New Britain
The largest island in the Bismarck Islands. It lie...
Osawa Pond - Osawa Pond
A pond located to the east of Daikakuji Temple in...
Abuse of the right to prosecute - kosokenranyo
When a prosecutor abuses his/her power to indict (...
Flavin adenine dinucleotide
...Abbreviation for flavin adenine dinucleotide. ...
Hansel and Gretel - Hänsel und Gretel
This is the 15th story in Grimm's Fairy Tales...
Young people's dance - Wakashuodori
(1) A type of Furyu dance that was popular in Kyot...
Pickled overnight - Ichiyazuke
〘 noun 〙① Turnips, greens, etc., finely chopped an...
Delhi Sultanate
The Sultanate of Delhi was a Muslim dynasty that r...









