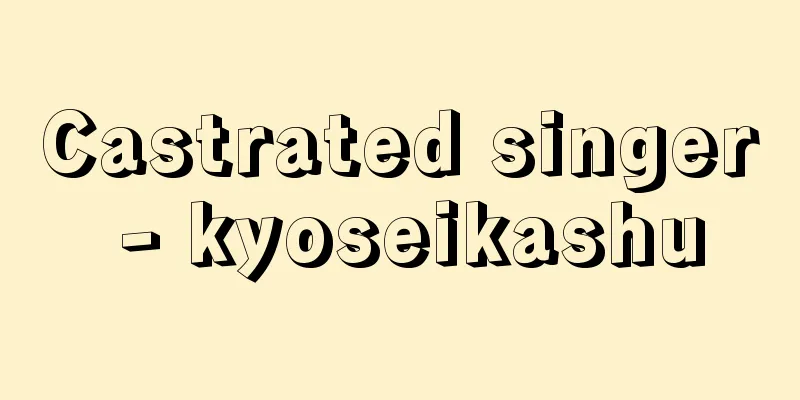Polar molecule

|
A general term for molecules with a non-zero dipole moment. Also called polar molecules. The dipole moment is the vector sum of the bond moments of each bond in a molecule. The bond moment is a vector quantity that occurs when the centers of gravity of the positive and negative charges of atoms A and B are apart. If the positive charge +e and the negative charge -e are at a distance of r, the bond moment μ AB is defined as e×r. If A and B are made of the same atom, the centers of gravity of the charges coincide with each other in both positive and negative, so r is zero and no bond moment occurs. However, if A is a hydrogen atom and B is a chlorine atom (hydrogen chloride molecule), the chlorine atom has more electrons than the hydrogen atom, so the center of gravity of the positive charge is biased toward the hydrogen atom and the center of gravity of the negative charge is biased toward the chlorine atom, so r is no longer zero and a dipole moment occurs. If there are multiple bonds with bond moments, the vector sum of the moments of each bond is the dipole moment of the molecule. In the water molecule, H2O , the two OH bonds have a bond moment, and the HOH angle is about 104 degrees, so the molecule is a polar molecule with a dipole moment. In contrast, in carbon tetrachloride, CCl4 , the carbon-chlorine bond has a bond moment, but the sum of the four vectors is zero, so the molecule as a whole has no dipole moment, making it a nonpolar molecule. Among compounds, nonpolar molecules are extremely limited. Typical nonpolar molecules include hydrocarbons such as methane, ethane, ethylene, and acetylene, aromatics such as benzene and naphthalene, carbon disulfide, and carbon tetrachloride. [Takashi Shimozawa] When a positive charge + and a negative charge - are at a distance Bond Moment Source: Shogakukan Encyclopedia Nipponica About Encyclopedia Nipponica Information | Legend |
|
双極子モーメントがゼロでない分子の総称。有極性分子ともいう。双極子モーメントは分子の中の各結合のもつ結合モーメントのベクトル和である。結合モーメントとは、原子Aと原子Bとの正電荷・負電荷の重心が離れているときに生ずるベクトル量である。いま、正電荷+eと負電荷-eとがrの距離にあるとすれば、結合モーメントμABはe×rで定義される。A、Bが同じ原子からなる場合は、電荷の重心は正負とも一致するのでrがゼロとなり、結合モーメントは生じない。しかし、Aが水素原子、Bが塩素原子(塩化水素分子)のようであれば、塩素原子の電子数が水素原子より多いので、正電荷の重心は水素原子側へ、負電荷の重心は塩素原子の側に偏り、rがゼロでなくなり、双極子モーメントが生ずる。結合モーメントをもつ結合が複数あれば、各結合のモーメントをベクトル和したものが分子の双極子モーメントとなる。水分子H2Oは二つのOH結合が結合モーメントをもち、角HOHが約104度なので、分子は双極子モーメントをもつ極性分子である。これに対して四塩化炭素CCl4は、炭素‐塩素の結合が結合モーメントをもつのに四つのベクトル和をとるとゼロになるので、分子全体の双極子モーメントがない無極性分子である。 化合物のなかで、無極性分子はきわめて限られている。メタン、エタン、エチレン、アセチレンなどの炭化水素、ベンゼン、ナフタレンなどの芳香族、二硫化炭素、四塩化炭素などが典型的な無極性分子である。 [下沢 隆] 正電荷+と負電荷-とがの距離にある場合©Shogakukan"> 結合モーメント 出典 小学館 日本大百科全書(ニッポニカ)日本大百科全書(ニッポニカ)について 情報 | 凡例 |
<<: Polar solvent - polar solvent
Recommend
Chloroprene rubber
A type of synthetic rubber. It is produced by emu...
Oyama-shuku
...It is within commuting distance of Tokyo, abou...
Ricaniidae
…As their elegant name suggests, this group of he...
silverweed
…It is found in Europe, Siberia, China, Korea and...
Persian lilac (English spelling)
…It is also often planted as a garden tree or roa...
When?
A Zen monk of the Obaku sect in the early Edo per...
Rock Crab - Rock Crab
→Jishibari Source : Heibonsha Encyclopedia About M...
Cow hippopotamus - Ushikaba
An evergreen shrub of the family Ilex (APG classi...
Nocturne (English spelling) nocturne English
The title of a Romantic piece of music with a med...
Aman andom - Aman andom
...In urban areas, residents were organized into ...
Isawa [town] - Isawa
An old town in Higashiyatsushiro District, Yamanas...
Orchis (English spelling)
A deciduous terrestrial orchid belonging to the Or...
Industrial Union
→Industry-specific unions Source: Shogakukan Ency...
Fold - Flat
It is a type of mo, a type of clothing worn by th...
Gasenshi (drawing paper)
A Japanese version of Chinese calligraphy paper. O...