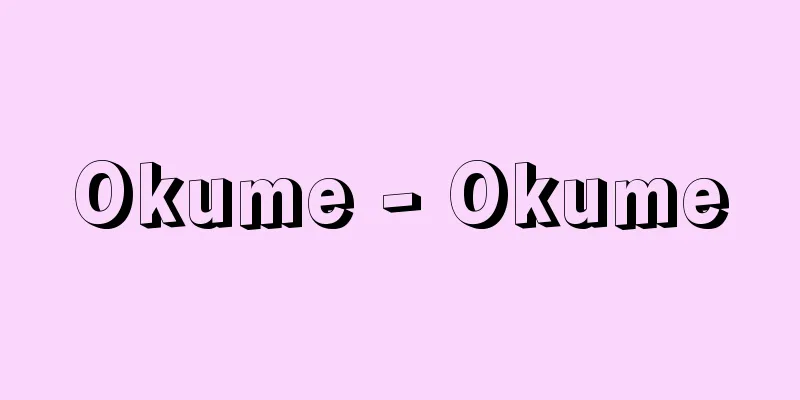Organic compound - organic compound

|
It is a general term for carbon compounds, excluding a few simple compounds such as carbon monoxide, carbon dioxide, carbonates, hydrogen cyanide and its salts, and carbon disulfide. It is often simply called organic matter. The term "organic compound" was first used by the Swedish scientist Berzelius in 1806. At that time, it was believed that compounds obtained from the natural animal and plant kingdoms as biological components, excretions, and fermentation products were essentially different from inorganic compounds (inorganic substances) obtained from the mineral kingdom, and that they were produced in the organs of living organisms by the vitality of the organism, so the name "organic" was given to them. It was believed that organic compounds were produced by the vitality of the organism and that it was impossible to produce them from inorganic compounds in a laboratory, but in 1828, the German scientist Wöhler synthesized the organic compound urea by heating ammonium cyanate, which was known as an inorganic compound, and the essential difference between organic compounds and inorganic compounds disappeared. Prior to this, the French scientist Lavoisier had shown through combustion experiments that organic compounds contained carbon and hydrogen, and the idea that organic compounds were carbon compounds was established. However, a small number of carbon compounds, such as carbon oxides, carbonates, and cyanide compounds, which had been considered inorganic compounds before Lavoisier, are still classified as inorganic compounds today. Organic compounds are based on hydrocarbons, which are composed of covalent bonds between carbon and carbon and between carbon and hydrogen. Many carbon atoms can be covalently bonded together to form chains or rings, and carbon-carbon bonds can be double or triple bonds in addition to single bonds, so there are many hydrocarbons, which are compounds of carbon and hydrogen. Furthermore, by substituting functional groups containing various heteroatoms such as oxygen, nitrogen, and sulfur at various positions in the hydrocarbon skeleton, many more derivatives can be produced, resulting in a very large number of organic compounds, with several million known, and this number is still increasing ( ). Although the properties of organic compounds vary considerably depending on the type, they generally share the common properties of being flammable, having a low melting point compared to inorganic substances, being less soluble in water, and being easily soluble in organic solvents such as alcohol and ether. In order to give names to each of the many organic compounds according to their chemical structure, the International Union of Pure and Applied Chemistry (IUPAC) has established a nomenclature system for organic compounds, which allows organic compounds to be named systematically. [Mr. Hirota November 18, 2016] "Fine Chemicals: Structure and Properties of Organic Compounds" by Mitsuhiko Tobita and Yasuzo Uchida (1982, Maruzen) " "Easy-to-understand Organic Chemistry: Organic Compounds for Living Organisms and Materials" by Satoshi Kobe, Akira Takamasa, and Mitsuji Saito (1994, Kodansha) [References] | | | | | |©Shogakukan "> Classification of organic compounds (diagram) Source: Shogakukan Encyclopedia Nipponica About Encyclopedia Nipponica Information | Legend |
|
一酸化炭素、二酸化炭素、炭酸塩、シアン化水素とその塩、二硫化炭素など少数の簡単な化合物を除く炭素化合物の総称である。単に有機物ということも多い。最初に「有機化合物」という語を使ったのはスウェーデンのベルツェリウスで1806年のことであるが、当時は、天然の動植物界から生体成分、排出物、発酵生成物などとして得られる化合物が、鉱物界から得られる無機化合物(無機物)と本質的に異なり、生物の生命力によりその器官organでつくられると考えられていたので、有機organicの名が与えられた。有機化合物は生命力によって生成し、実験室で無機化合物からつくることは不可能であると信じられていたが、1828年にドイツのウェーラーが、無機化合物として知られていたシアン酸アンモニウムを加熱して有機化合物である尿素を合成したので、有機化合物と無機化合物の本質的な相違はなくなった。それ以前にフランスのラボアジエが、有機化合物が炭素、水素を含むことを燃焼の実験により示していたので、有機化合物は炭素化合物であるとの考え方が確立されてきたが、ラボアジエ以前から無機化合物とされてきた酸化炭素、炭酸塩、シアン化合物など少数の炭素化合物は現在でも無機化合物として分類されている。 有機化合物の基本は炭化水素であり、炭素と炭素、炭素と水素の共有結合で構成されている。炭素どうしは共有結合により多数が結び付いて鎖状や環状の骨格をつくれるうえ、炭素‐炭素の結合には単結合のほかに二重結合や三重結合も可能であるので、炭素と水素の化合物である炭化水素だけでも多数存在する。そのうえ、炭化水素骨格のいろいろな位置を酸素、窒素、硫黄(いおう)などのいろいろなヘテロ原子を含む官能基で置換するとさらに多数の誘導体ができるので、有機化合物の数は非常に多数となり、数百万が知られているともいわれ、現在でもその数は増加しつつある()。有機化合物といっても、その種類により性質はかなり異なっているが、一般に可燃性であり、無機物に比べて融点が低く、水に溶けにくくアルコール、エーテルなどの有機溶媒に溶けやすいという共通の性質をもっている。数多い有機化合物の一つ一つにその化学構造に対応して名前をつけるために、国際純正・応用化学連合(IUPAC:International Union of Pure and Applied Chemistry)による有機化合物命名法が定められていて、これによると有機化合物を系統的に命名できる。 [廣田 穰 2016年11月18日] 『飛田満彦、内田安三著『ファインケミカルズ 有機化合物の構造と物性』(1982・丸善)』▽『神戸哲・高昌晨晴・斉藤光司著『わかりやすい有機化学――生体と材料のための有機化合物』(1994・講談社)』 [参照項目] | | | | | |©Shogakukan"> 有機化合物の分類〔図〕 出典 小学館 日本大百科全書(ニッポニカ)日本大百科全書(ニッポニカ)について 情報 | 凡例 |
>>: Organic analysis - organic analysis
Recommend
"Clumsy Left Carving" - Clumsy Left Carving
...The previous year at the Kawarazakiza Theater,...
Song sound - Soon
〘Noun〙 A type of Chinese character pronunciation. ...
Odoro - Odoro
... Doro-doro: A sound effect-like musical accomp...
Ermesinde
...The County of Luxembourg was established in th...
Ujitawara [town] - Ujitawara
A town in Tsuzuki County in the southern part of K...
Craftsman - Shokunin
A craftsman is someone who produces handmade good...
Smoke tree
A deciduous shrub or small tree of the Anacardiace...
Usuitaboya - Usuitaboya
…The zoonotes have 9–16 or 10–17 gill slit rows. ...
Ascension of Christ - Christ's Shoten (English spelling) Ascension
A theme in Christian art. This iconography, which ...
Kyoto Red Flag Incident
…High Treason Incident (2) An incident that occur...
Cooper, T.
…As this “Encyclopaedia” symbolizes, the 16th and...
Gustav Landauer
Born: April 7, 1870, Karlsruhe Died May 2, 1919, M...
Huán rén (English spelling)
Currently, this area is in the area of Hunjin Co...
abrasion
…The soil and sand carried by flowing water usual...
Precision machinery industry
This is an industry that produces instruments tha...





![Urmia [Lake] - Urmia](/upload/images/67cf930815c57.webp)