Mass number - mass number
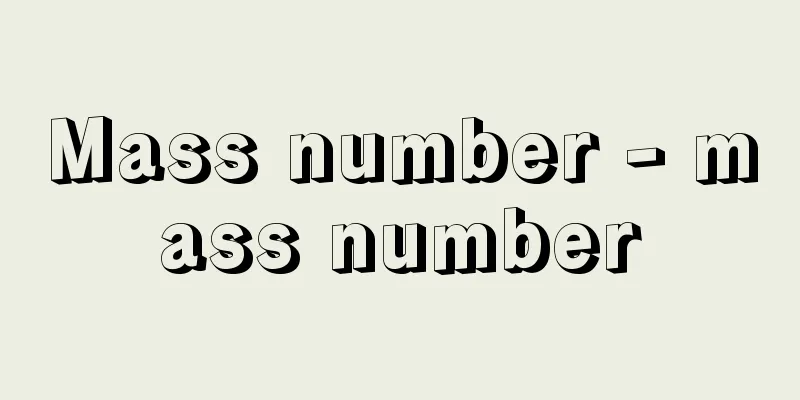
|
The protons and neutrons that make up an atomic nucleus are collectively called nucleons, and their total number is called the mass number. The nucleus, located at the center of an atom, is about 1/5000th the size of the electrons that surround it (i.e., the size of the atom). If we consider an atom with a diameter of several hundred meters, the central nucleus is barely the size of a ping-pong ball. However, most of the mass of an atom is due to the mass of the nucleus. It became known that neutrons, discovered by Chadwick in 1932, have the same mass as protons and make up the atomic nucleus together with protons. Thus, the mass of an atomic nucleus is almost an integer multiple of the mass of the nucleons (protons and neutrons), except for about 1% of the mass defect, so this integer can be used to distinguish between them in terms of mass in kilograms. In other words, if the number of protons is Z and the number of neutrons is N , the mass number A is equal to the sum of the two, and can be expressed as A = Z + N. In an atom, the number of electrons is the same as the proton number Z , and the atomic symbol is used to represent the atomic nucleus, but the mass number must be specified separately. It is customary to write the mass number in the upper left corner of the atomic symbol and the atomic number (number of protons) in the lower left, so for example, the nucleus of carbon, which consists of 6 protons and 6 neutrons (mass number 6 + 6 = 12), is written as 12 6 C. [Koji Bando and Toshio Motoba] Source: Shogakukan Encyclopedia Nipponica About Encyclopedia Nipponica Information | Legend |
|
原子核を構成している陽子と中性子をあわせて核子とよび、それらの総数を質量数という。原子の中心に位置する原子核は、周辺を遠巻きにしている電子群の大きさ(すなわち原子の大きさ)に比べて約5000分の1という微小さである。仮に直径数百メートルの原子を考えたとき、中心の原子核はやっとピンポン玉程度の大きさに相当する。しかし、原子の質量のほとんどは原子核の質量によるものである。チャドウィックによって1932年に発見された中性子は、陽子と同程度の質量をもっていて、陽子とともに原子核を構成していることが知られるようになった。こうして原子核の質量は、約1%の質量欠損を除き、核子(陽子と中性子)の質量のほぼ整数倍になっているので、質量何キログラムというかわりにこの整数を用いて区別することができるのである。すなわち、陽子の数をZ、中性子の数をNとすると、質量数Aは両者の和に等しくA=Z+Nで表される。原子にあっては電子の数は陽子数Zと同じであり、その原子核を表すのにも元素記号を用いるが、質量数は別途指定することが必要となる。質量数は原子記号の左上に、原子番号(陽子の数)は左下に書く習慣になっているので、たとえば、6個の陽子と6個の中性子から構成される炭素(質量数6+6=12)の原子核は126Cと書き表す。 [坂東弘治・元場俊雄] 出典 小学館 日本大百科全書(ニッポニカ)日本大百科全書(ニッポニカ)について 情報 | 凡例 |
Recommend
Ecole Executive - Ecole Executive
…The Commentary School of Law spread throughout E...
Bancroft, R.
…The English Reformation followed a different pat...
Ship bottom paint - Senteitoryou
Paint applied to the bottom of a ship below the l...
Kawachi administration
...In addition, the existence of the first 9 empe...
Fish price - Gyoka
Fish prices refer not only to the price of fish, b...
Juan Donoso Cortés
1809‐53 Spanish politician and diplomat. A central...
Alomicin
...Aloe contains over a dozen ingredients, includ...
Collection of famous things from ancient times - Kokonmeibutsu Ruiju
An illustrated guide to famous tea utensils compil...
Oyamaji - Oyamaji
A temple in Sakuragawa City, Ibaraki Prefecture. I...
Tatsunoichi
An ancient market in the area adjacent to Daian-ji...
Grosser katechismus (English spelling)
…It was written by taking the tradition of faith ...
Osei Tutu - Osei Tutu
…Until the second half of the 17th century, the A...
Takamitsu Awataguchi - Awataguchi Ryuko
Year of birth: Year of birth and death unknown. A ...
Chartres Cathedral - Cathédrale Notre-Dame de Chartres
A typical Gothic cathedral in France. Built in Ch...
Labé, Louise
[Born] Around 1524 [Died] 1566 A French female poe...