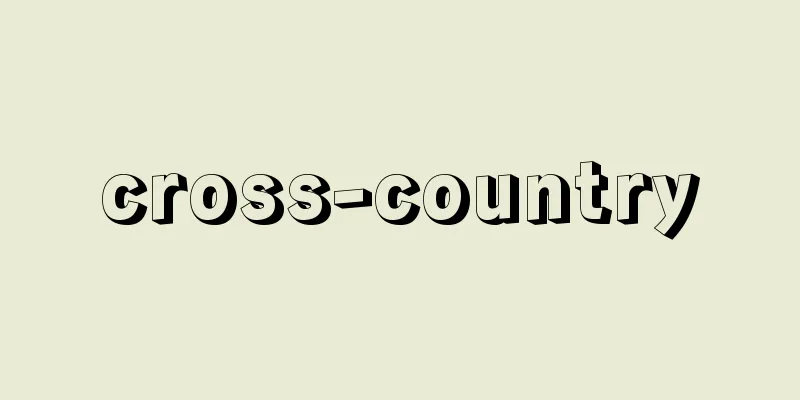Sodium cyanide

|
A cyanide of sodium. It is also called sodium cyanide or sodium cyanide. Traditionally, the main method of production was the Castner method, which uses metallic sodium, ammonia, and charcoal as raw materials, but in recent years, hydrocyanic acid (hydrocyanic acid) has become available cheaply and in large quantities, so a method of directly absorbing it into a sodium hydroxide solution has been used. HCN + NaOH -> NaCN + H2O [Torii Yasuo] [References] | [Additional information] |©Shogakukan "> Treatment of cyanide waste liquid Source: Shogakukan Encyclopedia Nipponica About Encyclopedia Nipponica Information | Legend |
|
ナトリウムのシアン化物。青酸ソーダ、青酸ナトリウムなどといわれる。従来は金属ナトリウム、アンモニアおよび木炭を原料とするカストナー法などが製造法の主流であったが、近年ではシアン化水素酸(青酸)が安価、多量に得られるようになったので、これを直接水酸化ナトリウム溶液に吸収させる方法が行われている。 HCN+NaOH―→NaCN+H2O [鳥居泰男] [参照項目] | [補完資料] |©Shogakukan"> シアン廃液の処理法 出典 小学館 日本大百科全書(ニッポニカ)日本大百科全書(ニッポニカ)について 情報 | 凡例 |
<<: Cyanic acid (English spelling)
Recommend
Eidenshi - Eidenshi
…At first, there were many in northern China, and...
Ornithischia
…A.S.Romer (1950) divided them into five orders: ...
Veneti
…the ancestors of the Western Slavs. In Latin, th...
Odo
…This monastery was founded in 909-910 by William...
Wilhelm Meister Theory
…F. Schlegel's Athenaeum Fragments, Idea Frag...
Hon Mikage - Hon Mikage
The name of a granite stone that has long been qu...
Business education - jitsugyokyoiku
A general term for education for people working in...
Liberation (Russia)
…It was founded in Moscow in October 1905, mainly...
Flaccus Albinus (English spelling) FlaccusAlbinus
…theologian and writer at the court of Charlemagn...
Colored thread embroidery - Iroito Shishuu
…〈Sashi〉 means to sew with a needle, and 〈繡〉 mean...
Occult blood test
…This type of bleeding is called occult bleeding....
Equal name - Kintomyo
A manor consisting of roughly equal sizes of arou...
Irekobishi - Irekobishi
A type of woven pattern with diamonds placed insid...
Nosema
...The malaria parasite Plasmodium is transmitted...
Kingdom of Castile and Leon
…Politically, at the beginning of the Reconquista...