Silver oxide battery

|
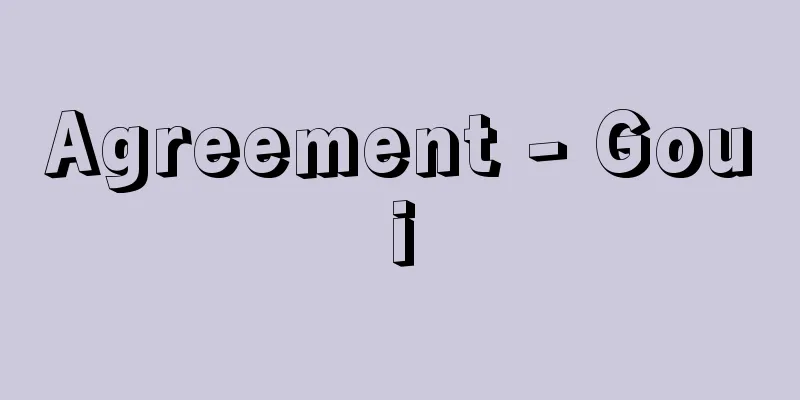
This button-type alkaline primary battery uses monovalent silver oxide Ag2O powder with a conductive agent added as the positive electrode active material, zinc powder mixed with a gelling agent as the negative electrode active material, and a highly concentrated 20-45% aqueous solution of potassium hydroxide KOH or sodium hydroxide NaOH with added zinc oxide ZnO as the electrolyte. It was developed in the United States in the 1960s. Using KOH as the electrolyte provides good high-rate discharge characteristics and low-temperature discharge characteristics. Using NaOH reduces these characteristics slightly, but improves leakage resistance. The electromotive reaction is (positive electrode) When divalent silver oxide (AgO) is used as the positive electrode active material, the discharge capacity per unit weight can be increased because the oxidation number is 2. However, in the positive electrode, 2AgO+H 2 O+2e - ―→Ag 2 O+2OH - Silver oxide button batteries are used in electronic watches, cameras, calculators, electronic toys, and electronic thermometers, but because silver is expensive, their market share is gradually being taken over by alkaline button batteries and button lithium batteries. Silver-zinc oxide batteries can also be used as storage batteries, but because they are expensive and have a short charge-discharge cycle life, they are limited to use as a power source for special applications such as space development, marine development, and military use, where their characteristics of high output and high energy density can be utilized, and production volumes are low. [Mitsuru Asano] "Electrochemistry" edited by Zenpachi Ogumi (2000, Ohmsha)" ▽ "Electrochemical Society, ed., "Electrochemistry Handbook" (2000, Maruzen)" ▽ "Battery Handbook" edited by the Battery Handbook Editorial Committee (2001, Maruzen)" [Reference] | | | |©Shogakukan "> Silver oxide battery structure Source: Shogakukan Encyclopedia Nipponica About Encyclopedia Nipponica Information | Legend |
|
正極活物質には1価の酸化銀Ag2O粉末に導電剤を加えたもの、負極活物質には亜鉛粉末にゲル化剤を混合したものを用い、電解液には酸化亜鉛ZnOを添加した20~45%の高濃度の水酸化カリウムKOHあるいは水酸化ナトリウムNaOH水溶液を用いたボタン形アルカリ一次電池。1960年代にアメリカで開発された。電解液にKOHを用いると高率放電特性や低温放電特性がよい。NaOHを用いるとそれらの特性はすこし劣るが耐漏液性を向上することができる。起電反応は 2価の酸化銀AgOを正極活物質に用いると、酸化数が2であるので単位重量当りの放電容量を増すことができる。しかし正極では ボタン形酸化銀電池は電子式腕時計やカメラ、電卓、電子玩具、電子体温計などに用いられているが、銀が高価であるためそのシェアはアルカリボタン電池やボタン形リチウム電池などにしだいに奪われてきている。 酸化銀亜鉛電池は蓄電池として利用することもできる。しかし高価で充放電サイクル寿命が短いため、その高出力、高エネルギー密度の特徴を生かすことができる宇宙開発、海洋開発、軍事用などの特殊な用途の電源に限られ、生産量は少ない。 [浅野 満] 『小久見善八編著『電気化学』(2000・オーム社)』▽『電気化学会編『電気化学便覧』(2000・丸善)』▽『電池便覧編集委員会編『電池便覧』(2001・丸善)』 [参照項目] | | | |©Shogakukan"> 酸化銀電池の構造 出典 小学館 日本大百科全書(ニッポニカ)日本大百科全書(ニッポニカ)について 情報 | 凡例 |
Recommend
Morioka Domain
During the Edo period, this was a Tozama clan tha...
Acetyl CoA - Acetyl CoA
C 23 H 38 N 7 O 17 P 3 S (809.58). Abbreviated as...
Car tribe - Family
...The Miao and Yao (both collectively known as t...
Cappelletti - Cappelletti, Giovanni Vincenzo
Year of death: circa 1887 (circa 1887) Year of bir...
Loewi, O. (English spelling) LoewiO
...In the body, it is synthesized from choline an...
Grallina cyanoleuca (English name) Grallina cyanoleuca
…A species of bird in the family Grallina, or a g...
Thermal burn
…It is also called a burn, and medically known as...
Daigensui Myoo - Daigensui Myoo
(Daigensui is a translation of Āṭavaka. For exampl...
"Oushuu Baitsumado Obihiki" - Oushuu Baitsumado Obihiki
...However, since it was considered inappropriate...
Sedillot, CE (English spelling) SedillotCE
…They range from single-celled organisms with siz...
Sun Yijang - Son Yijang
A Chinese scholar in the late Qing Dynasty. His c...
The Theory of Island Biogeography
…Born in Alabama, he earned his PhD at Harvard Un...
Strabismus - Shashi (English spelling)
◎ One eye is misaligned [What kind of disease is i...
Kumano betto
The chief who governed and managed the Kishu Kuman...
Albert Memorial Chapel
…St. George's Chapel was built for the Knight...


![Ogoe [town] - Ogoe](/upload/images/67cb151363a43.webp)