Hydrolysis constant - Hydrolysis constant
|
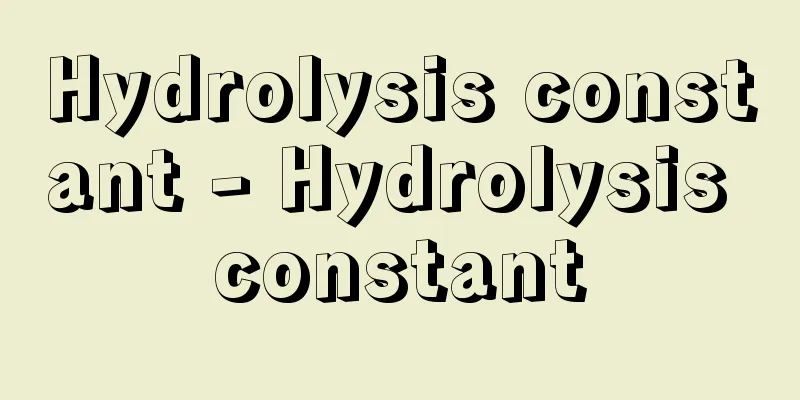
The equilibrium constant for the hydrolysis of the conjugate acid (or conjugate base) of a weak base produced by the ionization of the salt produced by the neutralization of a weak base and a strong acid (or a weak acid and a strong base, or a weak base and a weak acid). The NH 4 + produced by the neutralization of the weak base NH 3 with the strong acid HCl is, NH 4 + + H 2 O NH 3 + H 3 O + If the hydrolysis constant is K h , then Source: Morikita Publishing "Chemical Dictionary (2nd Edition)" Information about the Chemical Dictionary 2nd Edition |
|
弱塩基と強酸(または弱酸と強塩基,弱塩基と弱酸)の中和で生じた塩の電離で生じる弱塩基の共役酸(または弱酸の共役塩基)が加水分解する反応の平衡定数.弱塩基 NH3 と強酸HClの中和で生じる NH4+ は, NH4+ + H2O NH3 + H3O+ の加水分解を行う.加水分解定数を Kh とすれば, 出典 森北出版「化学辞典(第2版)」化学辞典 第2版について 情報 |
<<: Summer fortune-telling - Kazuiho
>>: Hydrolytic rancidity - Hydrolytic rancidity
Recommend
General memorandum for the Kyoto Imperial Government Office
A manual for the work of the Kyoto magistrate. Sev...
Musa coccinea (English spelling) Musacoccinea
… [Akira Takahashi]. … *Some of the terminology t...
MeV - Meev
Abbreviation for mega electron volt. 10 6 eV. Als...
Hawthorn - hawthorn
A deciduous shrub of the Rosaceae family (APG cla...
Xipacná (English spelling) Xipacna
…(2) Legends of giants as violent ancient gods wh...
Faras
...According to popular belief, the two kingdoms ...
Battle of Chaeronea - Battle of Chaeronea
A battle fought in Chaeronea, a city in northweste...
Superman
A 20th century American comic book superhero. He f...
Synodus macrops (English spelling) Synodus macrops
…[Kawaguchi Koichi]. … *Some of the terminology t...
Adi Buddha - Aadi Buddha
...Buddha as the origin of the universe. It is us...
Girton College
...With the intervention of Parliament, the requi...
Svedberg
Swedish physical chemist. Studied at Uppsala Univ...
Gyominkai - Gyominkai
…In 1921, Eizo Kondo, members of the Kyominkai an...
Industrial Design
It refers to the creative activity of planning an...
Graves, RJ
...A disease characterized by goiter, exophthalmo...

![Otowa [town] - Otowa](/upload/images/67cb2028d86b7.webp)






![Akitsu [town] - Akitsu](/upload/images/67cad16c22202.webp)
