Electrolysis

|
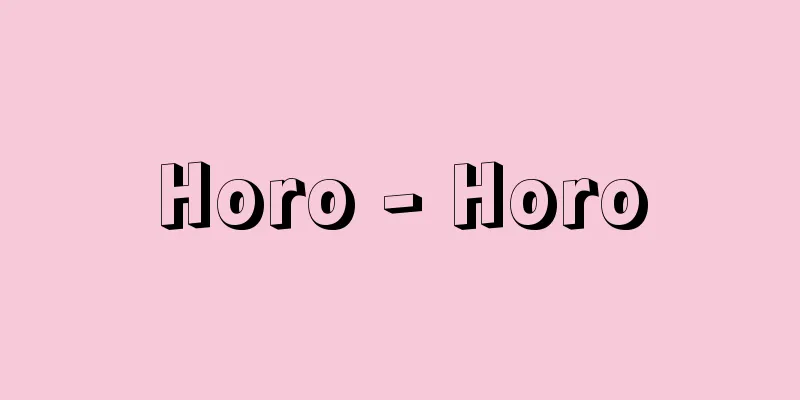
This refers to passing an electric current through an ionic conductor, such as an electrolyte solution or molten salt, to cause a chemical change. It is also called electrolysis for short. In practice, to pass an electric current, a pair of electrodes (rod- or plate-shaped conductors made of metal or graphite) are inserted into the ionic conductor and a DC power source is connected to them. When a current is passed through it, positive ions are electrically attracted to the electrode with the lower potential (called the cathode), and negative ions are electrically attracted to the electrode with the higher potential (called the anode), and the charges of the ions are neutralized (discharged) on the electrode surfaces, causing a chemical change. For example, when salt water is electrolyzed using platinum electrodes, In addition, when a pair of copper plate electrodes is placed in an aqueous solution of copper sulfate and electrolysis is performed, Electrolysis has many industrial applications, including electrolysis, electrometallurgy, electrolytic polishing, electroplating, and the manufacture of electrolytic capacitors, as well as the electrolysis of salt solutions to produce chlorine and caustic soda (sodium hydroxide). [Toda Genjiro] [Reference] | |©Shogakukan "> Electrolysis and batteries Source: Shogakukan Encyclopedia Nipponica About Encyclopedia Nipponica Information | Legend |
|
電解質水溶液あるいは溶融塩などのイオン伝導体に、電流を流して化学変化をおこさせることをいう。略して電解ともいう。実際には電流を流すためにイオン伝導体の中に1対の電極(金属や黒鉛などの棒状あるいは板状の導体)を挿入し、これに直流電源を接続する。電流を流すことにより、陽イオンはより電位の低いほうの電極(陰極という)のほうに、陰イオンは電位の高いほうの電極(陽極という)のほうに電気的に引かれて移動してきて、電極表面でイオンの電荷が中和され(放電)、化学変化をおこす。 たとえば、白金電極を用い食塩水を電解した場合、陽極では、 また、硫酸銅の水溶液中に1対の銅板電極をつけて電解すると、陽極上では、 電気分解は実際に工業的にも広く利用されている。そのなかには電解分析、電気冶金(やきん)、電解研摩、電気めっき、電解コンデンサーの製造など、また食塩水の電気分解による塩素およびカ性ソーダ(水酸化ナトリウム)の製造などがある。 [戸田源治郎] [参照項目] | |©Shogakukan"> 電気分解と電池 出典 小学館 日本大百科全書(ニッポニカ)日本大百科全書(ニッポニカ)について 情報 | 凡例 |
Recommend
Coelostat - Coelostat (English spelling)
A device that always sends light from the sun and...
Appearance theory - Gaikanriron
…It refers to the legal appearance in the German ...
Gill hole - gill hole
…In vertebrates, the anterior part of the digesti...
Zwickauer Propheten (English spelling)
...At the time, when central power was weak, many...
Tengu's Claws
A former Japanese name for fossilized shark teeth...
Toyota Motor Corporation - Toyota Motor Corporation
The world's top automobile manufacturer and Ja...
Battle of Dannoura
The final battle between the Genpei and Taira clan...
Onba - Onba
...In Kikaijima, Amami, they were called Okura an...
Isopyrum dicarpon (English spelling)
...It is distributed mainly in the eastern part o...
blastopore
…When an amphibian egg is fertilized, it divides ...
Alimenta
…Senatorial decisions were still important as a f...
Territorium
...usually refers to the partial states (lands) t...
Fussa [city] - Fussa
A city in western Tokyo. It was incorporated as a ...
Gate Theatre
Other playwrights with a strong connection to the...
Kanuma soil
It is a weathered pumice stone from a volcanic er...