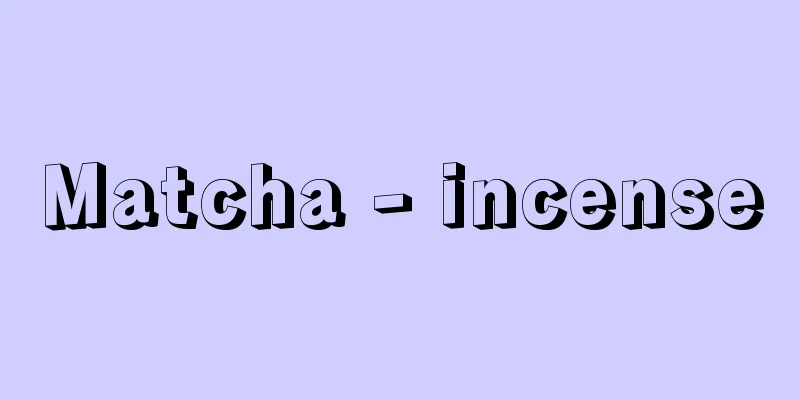Chromic acid

|
The oxoacid of chromium(VI), H 2 CrO 4 , has never been isolated. Because an aqueous solution of chromium(VI) oxide, CrO 3 , is a chromic acid solution, chromium(VI) oxide was sometimes called chromic acid. CrO3 + H2O → H2CrO4 2CrO 4 2- +2H + Cr 2 O 7 2- +H 2 O Source: Shogakukan Encyclopedia Nipponica About Encyclopedia Nipponica Information | Legend |
|
クロム(Ⅵ)のオキソ酸H2CrO4であるが、この酸自体は単離されていない。酸化クロム(Ⅵ)CrO3の水溶液がクロム酸溶液であるため、酸化クロム(Ⅵ)がクロム酸といわれたこともあった。 CrO3+H2O→H2CrO4 2CrO42-+2H+Cr2O72-+H2O 出典 小学館 日本大百科全書(ニッポニカ)日本大百科全書(ニッポニカ)について 情報 | 凡例 |
Recommend
Eboshigo - Eboshigo
〘Noun〙① In the past, a samurai boy was given an eb...
Ephemeral stream
A river that normally has no water. It can be a r...
IMF Position - IMF Position
…SDRs are international reserve assets that count...
Ordinary accounting - Normal accounting
The accounting of local governments is the sum of ...
Spreader - Tenchakuzai (English spelling)
An adjuvant used to strengthen the spreading power...
Convertibility of the yen
…(1) This allows nonresidents to freely exchange ...
Blood Daruma of Hosokawa - Blood Daruma of Hosokawa
A story of the origin of a famous treasure handed ...
Protoperidinium
... P . conicum and P. pentagonium are commonly f...
Tail - O
A protrusion at the rear end of an animal's b...
Gravitational waves - Gravitational waves
This phenomenon occurs when the movement of a mass...
Allium sphaerocephalum - Allium sphaerocephalum
...A few leaves usually develop in early spring, ...
Rejection syndrome
One of the most common psychomotor disorders in sc...
Aiakos
In Greek legend, he was the king of Phthia in The...
Art Institute of Chicago
Along with the Metropolitan Museum of Art and the...
Andree, EW
...It is located on the west bank of the Tigris R...