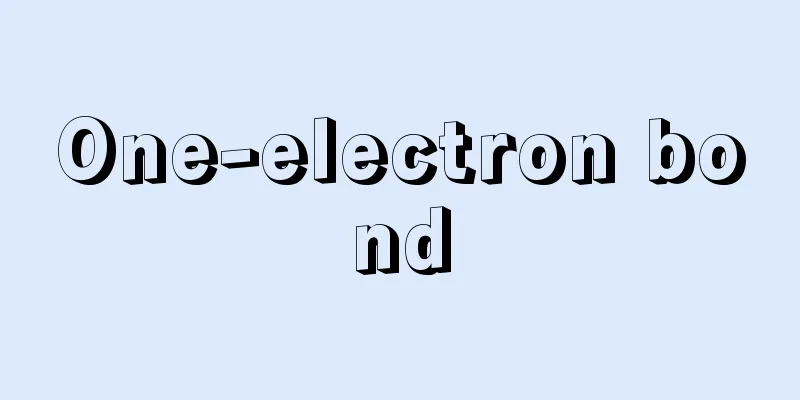
One-electron bond
|
This refers to a chemical bond formed between two atomic nuclei A and B when one electron is shared by both nuclei A and B. The dissociation energy of a one-electron bond is smaller than the dissociation energy of a normal covalent bond formed when two electrons pair up. A typical example of a one-electron bond is the hydrogen molecule ion H2 + , whose dissociation energy is 2.55 x 105 J/mol, which is about 60% of the dissociation energy of the hydrogen molecule H2, which is 4.27 x 105 J/mol. [Tasumi Mitsuo] Source: Heibonsha World Encyclopedia, 2nd Edition Information |
|
1個の電子が2個の原子核A,Bによって共有されることによってAB間に生じる化学結合を指す。一電子結合の解離エネルギーは,2個の電子が対をなすことによって形成される通常の共有結合の解離エネルギーよりも小さい。一電子結合の代表例は水素分子イオンH2+にみられ,その解離エネルギーは水素分子H2の解離エネルギー4.27×105J/molの約60%にあたる2.55×105J/molである。【田隅 三生】
出典 株式会社平凡社世界大百科事典 第2版について 情報 |
<<: Positional astronomy - Ichitenmongaku
Recommend
《Izari Katsugoro》 - Izari Katsugoro
…It is said that on January 21, 1590 (Tensho 18),...
Kleoboulos
…He is credited with writing maxims such as "...
Eunicidae
…A general term for annelids belonging to the fam...
Jagir - Jagir (English spelling)
Lands and fiefs in the Mughal Empire, India. The ...
Perspective drawing - Toushizu
This is a geometric projection that was created i...
Naigameṣa (English spelling)
…Among them, Gomukha (Yaksha), Ambikā and Rohiṇī ...
Virgo (the virgin)
Abbreviation: Vir. One of the twelve zodiacal sign...
Scribing - Scribbling
The act of marking a workpiece to be machined or h...
Spectrometry
...Therefore, the medium for measurement is not l...
Head of family register - Koseki hittousha
The first person listed in each family register. I...
Japanese bush warbler - Japanese bush warbler
…It is also planted as an ornamental garden tree....
Irish Citizen Army
…During World War I, the British tried to purchas...
Surikata (English spelling)
A carnivorous civet that often stands on its hind ...
Candler, A.G.
…Pemberton sold the concentrate to drug stores an...
System - system (English spelling)
It refers to a "whole" formed by each c...