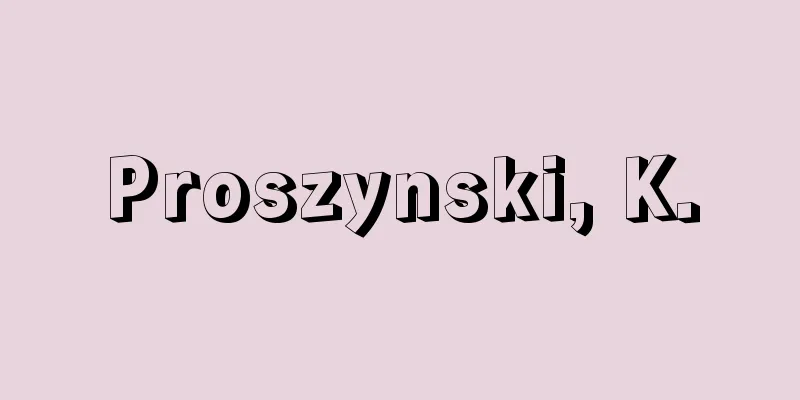Hydrodesulfurization - Hydrodesulfurization

|
A method of removing sulfur compounds contained in a substance by hydrogenating them and converting them to hydrogen sulfide. Currently, it is mainly used for desulfurization of petroleum fuels and petrochemical feedstocks. Since the removal of nitrogen, oxygen, halogens, and metals contained in these feedstocks and the hydrogenation and cracking of unsaturated hydrocarbons usually occur at the same time, it is also called hydrorefining in petroleum refining and hydrotreating in the synthesis of lubricating oils. In general, thiols (mercaptans) are the easiest to hydrogenate among organic sulfur compounds, followed by sulfides, disulfides, cyclic sulfur compounds, and thiophenes, in that order, and the higher the molecular weight of a substance, the more difficult it is to hydrogenate. The reaction conditions vary depending on the process and the raw materials being used, but the pressure is between 4 and 170 atmospheres, the temperature is between 290 and 450°C, and a catalyst such as cobalt-molybdenum sulfide or nickel-molybdenum sulfide supported on alumina (for stable maintenance) is used. As a measure to prevent air pollution, advanced desulfurization processes have been developed for gasoline and diesel fuels, and since 2005, gasoline and diesel fuel have been sulfur-free (sulfur content of 10 ppm or less. ppm = one part per million). This has also had a major effect on improving the performance and extending the life of exhaust gas purification catalysts. The hydrogen sulfide produced is oxidized and recovered as elemental sulfur. There are two recovery methods: gas phase and liquid phase. The gas phase method involves oxidizing part of the hydrogen sulfide to sulfur dioxide, which is then reacted with hydrogen sulfide (Claus reaction) to produce elemental sulfur. The liquid phase method involves absorbing hydrogen sulfide into a weak alkaline solution and oxidizing it in the solution using an oxidizing agent to produce elemental sulfur. The liquid phase method has a high recovery rate, but the purity of the recovered sulfur is higher with the gas phase method. [Takeaki Yashima] [Reference item] |©Shogakukan "> Hydrodesulfurization reaction Source: Shogakukan Encyclopedia Nipponica About Encyclopedia Nipponica Information | Legend |
|
物質中に含まれる硫黄(いおう)化合物を水素化し、硫化水素に転化して除去する方法。現在では、おもに石油系燃料および石油化学原料の脱硫に用いられる。通常はこれらの原料に含まれている窒素、酸素、ハロゲン、金属の除去および不飽和炭化水素の水素化、分解などが同時におこるため、石油精製においては水素化精製、潤滑油の合成では水素化処理ともよばれる。 有機硫黄化合物の水素化は、一般にチオール(メルカプタン)がもっとも容易で、以下、硫化物、二硫化物、環式硫黄化合物、チオフェンの順となり、また高分子量の物質ほど水素化されにくい。 反応条件は、プロセスにより、また対象とする原料によりそれぞれ異なるが、圧力4~170気圧、温度290~450℃、アルミナに担持(安定に保持)したコバルト‐モリブデン硫化物あるいはニッケル‐モリブデン硫化物系の触媒が用いられる。 大気汚染防止対策として、自動車用燃料であるガソリンおよびディーゼル軽油の高度脱硫プロセスが開発され、2005年(平成17)以降ガソリンおよびディーゼル軽油のサルファーフリー(硫黄分10ppm以下。ppm=100万分の1)が実現している。また、これによる排気ガス浄化触媒の高性能化および長寿命化の効果も大きい。 生成した硫化水素は、酸化され単体硫黄として回収される。回収法には気相法と液相法とがある。気相法は、硫化水素の一部を酸化して二酸化硫黄とし、硫化水素と反応(クラウス反応)させて単体硫黄とする方法である。液相法は、硫化水素を弱アルカリ溶液に吸収し、溶液中で酸化剤を用いて酸化して、単体硫黄とする方法である。液相法は回収率は高いが、回収硫黄の純度は気相法のほうが高い。 [八嶋建明] [参照項目] |©Shogakukan"> 水素化脱硫反応 出典 小学館 日本大百科全書(ニッポニカ)日本大百科全書(ニッポニカ)について 情報 | 凡例 |
<<: Sodium Hydride - Sodium Swiss
>>: Lithium aluminum hydride - Suisoka aluminum lithium (English spelling) aluminum lithium hydride
Recommend
Khmel'nitskii, Bogdan Mikhailovich
[Born] circa 1595. Chigirin? [Died] August 16, 165...
Cereal cultivation
This refers to an extensive, large-scale agricultu...
Pterois volitans (English spelling) Pteroisvolitans
...As it grows, the front half of its body turns ...
bande
...It means an instrumental ensemble, but today i...
Aztec Kingdom - Aztec Kingdom (English spelling)
An Indian (native) kingdom that flourished in Mexi...
Channel Islands
An archipelago in the English Channel south of the...
Naturalist
〘noun〙 (naturalist) ① A person who is interested i...
pension
...Subsequently, as travel demand became more div...
Iris wine - Ayamezake
〘Noun〙 Sake made by steeping cut roots and leaves ...
Habenaria
...A small orchid plant (illustration) that produ...
Sale (English spelling)
A town in central Myanmar, located about 50 km sou...
Vlajkov, TG (English spelling) VlajkovTG
…In Under the Yoke, Bazov, the father of national...
Obai - Obai
A deciduous shrub of the Oleaceae family (APG cla...
Marcello (English spelling) Marcello, Benedetto
Born: July 24, 1686, Venice Died July 24, 1739. It...
barometer
…An instrument for measuring atmospheric pressure...